Experimental Medical Treatments: Right To Try Law
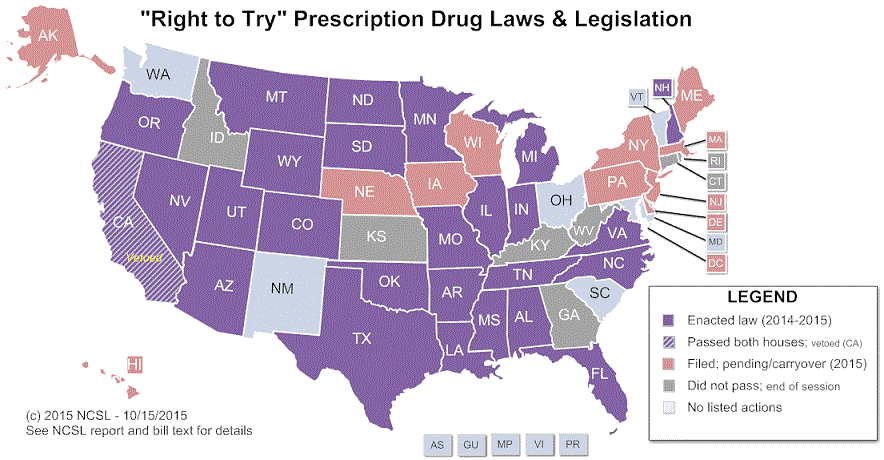
Prior to legislation, there were two primary options to access experimental therapies that have not yet been approved by the Food and Drug Administration (FDA). One is to participate in a clinical trial. The other is to apply to the FDA for access to the experimental drug, a process so cumbersome that it discourages many patients and physicians from applying. Right To Try laws are aimed at making experimental therapies more easily accessible to patients with terminal illnesses by removing the FDA application process and establishing expanded access programs for patients meeting certain eligibility requirements. Read the Michigan law and read a discussion about these laws.
Michigan and several other states have passed legislation intended to expand access to experimental treatments for patients with serious or life-threatening conditions